New material could improve safety for first responders to chemical hazards
30 Apr 2011
A new kind of sensor could warn emergency workers when carbon filters in the respirators they wear to avoid inhaling toxic fumes have become dangerously saturated.
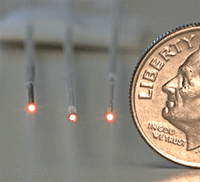 In a recent issue of the journal Advanced Materials, a team of researchers from the University of California, San Diego and Tyco Electronics describe how they made the carbon nanostructures and demonstrate their potential use as microsensors for volatile organic compounds.
In a recent issue of the journal Advanced Materials, a team of researchers from the University of California, San Diego and Tyco Electronics describe how they made the carbon nanostructures and demonstrate their potential use as microsensors for volatile organic compounds.
First responders protect themselves from such vapors, whose composition is often unknown, by breathing through a canister filled with activated charcoal – a gas mask.
Airborne toxins stick to the carbon in the filter, trapping the dangerous materials.
As the filters become saturated, chemicals will begin to pass through. The respirator can then do more harm than good by providing an illusion of safety. But there is no easy way to determine when the filter is spent. Current safety protocols base the timing of filter changes on how long the user has worn the mask.
''The new sensors would provide a more accurate reading of how much material the carbon in the filters has actually absorbed,'' said team leader Michael Sailor, professor of chemistry and biochemistry and bioengineering at UC San Diego. ''Because these carbon nanofibers have the same chemical properties as the activated charcoal used in respirators, they have a similar ability to absorb organic pollutants.''
Sailor's team assembled the nanofibers into repeating structures called photonic crystals that reflect specific wavelengths, or colors, of light. The wing scales of the Morpho butterfly, which give the insect its brilliant iridescent coloration, are natural examples of this kind of structure.


















