ORNL microscopy generates new view of fuel cells
17 Aug 2011
A novel microscopy method at the Department of Energy's Oak Ridge National Laboratory is helping scientists probe the reactions that limit widespread deployment of fuel cell technologies.
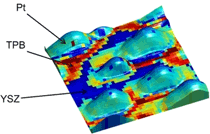 |
| A new ORNL microscopy technique allows researchers to study key reactions in fuel cells at an unprecedented scale. The overlay shows electrochemical activity of platinum (Pt) nanoparticles on an yttria-stabilized zirconia (YSZ) surface, revealing enhanced activity along the triple-phase boundaries (TPB). |
ORNL researchers applied a technique called electrochemical strain microscopy that enables them to examine the dynamics of oxygen reduction / evolution reactions in fuel cell materials, which may reveal ways to redesign or cut the costs of the energy devices. The team's findings were published in Nature Chemistry.
"If we can find a way to understand the operation of the fuel cell on the basic elementary level and determine what will make it work in the most optimum fashion, it would create an entirely new window of opportunity for the development of better materials and devices," said co-author Amit Kumar, a research scientist at ORNL's Center for Nanophase Materials Sciences.
Although fuel cells have long been touted as a highly efficient way to convert chemical energy into electrical energy, their high cost - in large part due to the use of platinum as a catalyst - has constrained commercial production and consumption.
Large amounts of platinum are used to catalyse the fuel cell's key reaction - the oxygen-reduction reaction, which controls the efficiency and longevity of the cell. Yet exactly how and where the reaction takes place had not been probed because existing device-level electrochemical techniques are ill suited to study the reaction at the nanoscale.
ORNL co-author Sergei Kalinin explains that certain methods like electron microscopy had failed to capture the dynamics of fuel cell operation because their resolution was effectively too high.



















