Promising new target for stifling the growth and spread of cancer
By By Scott LaFee | 14 Jun 2011
Cancer and chronic inflammation are partners in peril, with the latter increasing the likelihood that malignant tumours will develop, grow and spread.
Researchers at the University of California, San Diego School of Medicine, say they've identified a tumour inflammation trigger that is common to most, if not all, cancers.
Using existing inhibitory drugs, the scientists were able to dramatically decrease primary tumour growth in animal studies and, more importantly, halt tumour progression and metastasis.
The findings appear in the June 14 issue of the journal Cancer Cell, authored by Judith A. Varner, PhD, professor of medicine at the UC San Diego Moores Cancer Center, and colleagues in the UCSD School of Medicine and at the University of Torino, Italy.
When cancer cells appear in the body, they often provoke an immune system response.
Under some circumstances, this is a good thing. But Varner and colleagues were able to show that when responding myeloid or white blood cells called macrophages are drawn to invasive cancer cells, the result can be considerable trouble for patients.
Rather than suppressing the cancer, the myeloid cells are tricked by the tumour into aiding and abetting its growth and spread.
Scientists have long recognised that myeloid cells can invade and promote tumour growth. But until now it was not fully appreciated how this hijacking occurs and whether there are ways to disrupt this process by suppressing the trigger that leads to myeloid cell recruitment into tumours.
Probing more deeply into the tumour inflammation process, the UCSD research team identified a range of tumour-produced molecules that attract these dangerous myeloid cells.
They also pinpointed the specific trigger on myeloid cells enabling them to invade the tumour environment and accelerate tumour growth and metastasis. It is an enzyme called PI-3 kinase gamma on myeloid cells that turns on an adhesion receptor allowing the cells to enter tumours.
When researchers blocked the activity of PI-3-kinase-gamma, either genetically or through the use of a drug designed for this purpose, myeloid cells were blocked access into tumours, resulting in reduced tumour growth and a dramatic decrease in metastasis. Without the recruitment of myeloid cells, Varner said, the capability of a cancer tumour to grow is largely stifled.
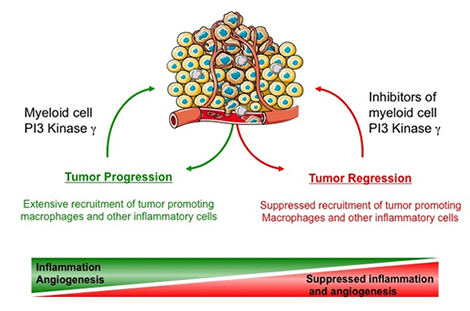 |
| Tumors are characterized by extensive inflammatory infiltrates, which can comprise up to 25 percent of the tumor's mass. Myeloid cells invade tumors in response to diverse inflammatory stimuli produced by the tumor. Invading myeloid cells differentiate into a type of macrophage that promotes tumor angiogenesis, growth and metastasis and inhibits anti-tumor immunity. In the June 14 issue of Cancer Cell, Schmid et al. demonstrate that tumor inflammation (myeloid cell invasion of tumors) requires PI3kinase gamma, a gatekeeper enzyme that is primarily expressed by myeloid cells. Inhibitors of PI3kinase gamma strongly inhibit tumor inflammation, growth and metastasis for a wide variety of cancers. PI3kinase gamma inhibitors hold promise as a new class of general cancer therapeutic agents. |
Tumour suppressor
Tumours are characterised by extensive inflammatory infiltrates, which can comprise up to 25 per cent of the tumour's mass. Myeloid cells invade tumours in response to diverse inflammatory stimuli produced by the tumour. Invading myeloid cells differentiate into a type of macrophage that promotes tumour angiogenesis, growth and metastasis and inhibits anti-tumour immunity.
In the 14 June issue of Cancer Cell, Schmid et al demonstrate that tumour inflammation (myeloid cell invasion of tumours) requires PI3kinase gamma, a gatekeeper enzyme that is primarily expressed by myeloid cells. Inhibitors of PI3kinase gamma strongly inhibit tumour inflammation, growth and metastasis for a wide variety of cancers. PI3kinase gamma inhibitors hold promise as a new class of general cancer therapeutic agents.
''Most strategies targeting the role of myeloid cells in cancer have focused on reducing their expression of inflammatory molecules,'' Varner explained. ''We've found a single convergent point – the PI-3 kinase-gamma enzyme – that, when blocked, appears to result in significant suppression of tumour inflammation and growth regardless of the initiating event. It could be a very important therapeutic target for future cancer treatments and could impact most, if not all, types of solid cancer.''
Michael Karin, PhD, distinguished professor of pharmacology in UCSD's Laboratory of Gene Regulation and Signal Transduction and a pioneer in inflammation research, agreed, ''I think that the inhibition of PI-3K activity represents a very interesting and promising approach for inhibition of tumor-associated inflammation. It seems to fully normalize the tumour microenvironment and provide a new addition to our armamentum of anti-cancer drugs.''
Varner said a number of biotechnology companies are pursuing potential drugs using PI-3-kinase inhibitors to treat diseases from cancer to heart disease to arthritis. The PI-3-kinase-gamma protein may be a particularly promising therapeutic target, because it is not widely expressed in the body, and its inhibition would likely produce fewer side effects than many therapeutics.
Funding for this research came, in part, from grants from the National Institutes of Health and the California Tobacco Related Disease Research Program.

















