The drive toward hydrogen vehicles just got shorter
22 Mar 2011
Researchers have revealed a new single-stage method for recharging the hydrogen storage compound ammonia borane. The breakthrough makes hydrogen a more attractive fuel for vehicles and other transportation modes.
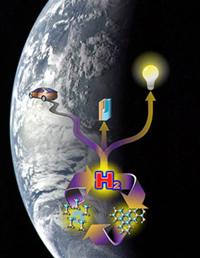 |
| An artist's conception of the one-pot method for transforming ammonium borane into hydrogen fuel and an illustration of how the technology, which doesn't produce greenhouse gasses, is good for the planet. |
In an article appearing today in the March 18 edition of Science magazine, Los Alamos National Laboratory (LANL) and University of Alabama researchers working within the US Department of Energy's Chemical Hydrogen Storage Center of Excellence describe a significant advance in hydrogen storage science.
Hydrogen is in many ways an ideal fuel. It possesses a high energy content per unit mass when compared to petroleum, and it can be used to run a fuel cell, which in turn can be used to power a very clean engine. On the down side, H2 has a low energy content per unit volume versus petroleum (it is very light and bulky). The crux of the hydrogen issue has been how to get enough of the element on board a vehicle to power it a reasonable distance.
Work at LANL and elsewhere has focused on chemical hydrides for storing hydrogen, with one material in particular, ammonia borane, taking center stage.
Ammonia borane is attractive because its hydrogen storage capacity approaches a whopping 20 percent by weight - enough that it should, with appropriate engineering, permit hydrogen-fueled vehicles to go farther than 300 miles on a single "tank," a benchmark set by the US Department of Energy.
Hydrogen release from ammonia borane has been well demonstrated, and its chief drawback to use has been the lack of energy-efficient methods to reintroduce hydrogen into the spent fuel once burned. In other words, until now, after hydrogen release, the ammonia borane couldn't be recycled efficiently enough.



















