Battery development may extend range of electric cars
11 Jan 2014
It's known that electric vehicles could travel longer distances before needing to charge and more renewable energy could be saved for a rainy day if lithium-sulphur batteries can just overcome a few technical hurdles. Now, a novel design for a critical part of the battery has been shown to significantly extend the technology's lifespan, bringing it closer to commercial use.
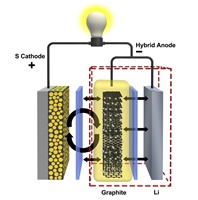 A "hybrid" anode developed at the Department of Energy's Pacific Northwest National Laboratory (PNNL) could quadruple the life of lithium-sulphur batteries. Nature Communications published a paper today describing the anode's design and performance.
A "hybrid" anode developed at the Department of Energy's Pacific Northwest National Laboratory (PNNL) could quadruple the life of lithium-sulphur batteries. Nature Communications published a paper today describing the anode's design and performance.
"Lithium-sulphur batteries could one day help us take electric cars on longer drives and store renewable wind energy more cheaply, but some technical challenges have to be overcome first," says PNNL Laboratory Fellow Jun Liu, who is the paper's corresponding author. "PNNL's new anode design is helping bringing us closer to that day."
Today's electric vehicles are commonly powered by rechargeable lithium-ion batteries, which are also being used to store renewable energy. But the chemistry of lithium-ion batteries limits how much energy they can store.
One promising solution is the lithium-sulphur battery, which can hold as much as four times more energy per mass than lithium-ion batteries. This would enable electric vehicles to drive longer on a single charge and help store more renewable energy. The down side of lithium-sulphur batteries, however, is they have a much shorter lifespan because they can't be charged as many times as lithium-ion batteries.
Most batteries have two electrodes - one is positively charged and called a cathode, while the second is negative and called an anode. Electricity is generated when electrons flow through a wire that connects the two. Meanwhile, charged molecules called ions shuffle from one electrode to the other through another path - the electrolyte solution in which the electrodes sit.
The lithium-sulfur battery's main obstacles are unwanted side reactions that cut the battery's life short. The undesirable action starts on the battery's sulphur-containing cathode, which slowly disintegrates and forms molecules called polysulphides that dissolve into the battery's electrolyte liquid.
The dissolved sulphur eventually develops into a thin film called the solid-state electrolyte interface layer. The film forms on the surface of the lithium-containing anode, growing until the battery is inoperable.
Most lithium-sulphur battery research to date has centered on stopping sulphur leakage from the cathode. But PNNL researchers determined stopping that leakage can be particularly challenging. Besides, recent research has shown a battery with a dissolved cathode can still work. So the PNNL team focused on the battery's other side by adding a protective shield to the anode.
The new shield is made of graphite, a thin matrix of connected carbon molecules that is already used in lithium-ion battery anodes. In a lithium-sulphur battery, PNNL's graphite shield moves the sulphur side reactions away from the anode's lithium surface, preventing it from growing the debilitating interference layer. Combining graphite from lithium-ion batteries with lithium from conventional lithium-sulphur batteries, the researchers dubbed their new anode a hybrid of the two.
The new anode quadrupled the lifespan of the lithium-sulphur battery system the PNNL team tested. When equipped with a conventional anode, the battery stopped working after about 100 charge-and-discharge cycles. But the system worked well past 400 cycles when it used PNNL's hybrid anode and was tested under the same conditions.
"Sulphur is still dissolved in a lithium-sulphur battery that uses our hybrid anode, but that doesn't really matter," Liu says. "Tests showed a battery with a hybrid anode can successfully be charged repeatedly at a high rate for more 400 cycles, and with just an 11-pe rcent decrease in the battery's energy storage capacity."
This and most other lithium-sulphur battery research is conducted with small, thin-film versions of the battery that are ideal for lab tests. Larger, thicker batteries would be needed to power electric cars and store renewable energy. Liu notes tests with a larger battery system would better evaluate the performance of PNNL's new hybrid anode for real-world applications.
This study was primarily supported by the Department of Energy's Office of Science (BES), with additional support from DOE's Advanced Research Projects Agency-Energy, and DOE's Office of Energy Efficiency and Renewable Energy. Some of this research was performed at EMSL, DOE's Environmental and Molecular Sciences Laboratory at PNNL.



















